accuracy vs precision in chemistry|Know Your Techniques: Accuracy, Precision, and : Tagatay Accuracy and precision are very similar in the fact, that they both refer to measurement quality, but they are very different indicators of . For inquiries, you may call our Metrobank Contact Center at (02) 88-700-700, or our domestic toll-free number at 1-800-1888-5775, or send an e-mail to
[email protected] Metrobank is regulated by the Bangko Sentral ng Pilipinas site: https://www.bsp.gov.ph
PH0 · What Is the Difference Between Accuracy and Precision?
PH1 · Know Your Techniques: Accuracy, Precision, and
PH2 · Difference Between Accuracy And Precision
PH3 · Accuracy, Precision, and Percent Error
PH4 · Accuracy vs. Precision in Chemistry
PH5 · Accuracy vs. Precision
PH6 · Accuracy and Precision
PH7 · 3.12: Accuracy and Precision
PH8 · 1.5: Measurement Uncertainty, Accuracy, and Precision
PH9 · 1.5 Measurement Uncertainty, Accuracy, and Precision
Planes & Seat Maps > Boeing 777-300ER (77W) SWISS Seat Maps. Boeing 777-300ER (77W) Overview; Planes & Seat Maps. Airbus A220-100 (CS1) Airbus A220-300 (CS3) Airbus A319-100 (319) Airbus A320-200 (320) . Swiss - is ONLY the name on the outside of this aircraft!seats are only about 43cm wide, meaning the passenger' shoulders and .
accuracy vs precision in chemistry*******In everyday speech, the terms accuracy and precision are frequently used interchangeably. However, their scientific meanings are quite different. Accuracy is a measure of how close a measurement is to the correct or accepted value of the quantity being measured. Precision is a measure of . Tingnan ang higit paThis action is not available. How do professional basketball players improve their shooting accuracy? Basketball is one of those sports where you need to hit the target. A . Tingnan ang higit paAccuracy is a measure of how close a measurement is to the correct or accepted value of the quantity being measured. Precision is a measure of how close a series of . Tingnan ang higit pa
Accuracy and precision are very similar in the fact, that they both refer to measurement quality, but they are very different indicators of . Accuracy is how close a value is to its true value. An example is how close an arrow gets to a bull's-eye center. Precision is how repeatable a measurement is. An example is. The first issue deals with the concept of accuracy while the second deals with precision. To illustrate these two concepts, consider an experiment in which the .
Both accuracy and precision are the goal of any measurement. Variations in accuracy and precision are largely controllable. In target shooting, you improve accuracy by moving .
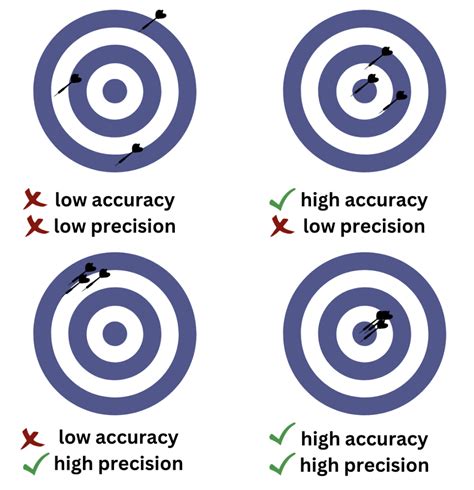
Accuracy and Precision. Scientists typically make repeated measurements of a quantity to ensure the quality of their findings and to evaluate both the precision and the accuracy of .Know Your Techniques: Accuracy, Precision, and Accuracy is how close a measurement is to the actual correct value. Precision is how close measurements are to each other. Accuracy is typically associated with . Accuracy and Precision. Scientists typically make repeated measurements of a quantity to ensure the quality of their findings and to know both the precision and the .Chemistry Measurement Accuracy, Precision, and Percent Error. Key Questions. How can precision be measured? Answer: Maybe by σd, the standard deviation.
Learn how to distinguish accuracy and precision in chemistry experiments and how to select the right equipment for your needs. Find out when to use top-loading or analytical balances, burets, pipettes, and other . This means its mass lies between 6.722 and 6.724 grams, an uncertainty of 0.001 gram. Every measurement has some uncertainty, which depends on the device used (and the user’s ability). All of the digits in a measurement, including the uncertain last digit, are called significant figures or significant digits.Accuracy is the quality that a measurement has if it is close to some other quantity’s true value. Precision shows how much a repeated measurement will change its value. For example, if several darts were thrown at a dart board, and all hit the same spot far away from the bull’s-eye, we could say the throws were precise, but inaccurate. If the darts .Chem 1402: General Chemistry 1 (Belford) Text 1.B: Review of the Tools of Quantitative Chemistry . There are two concepts we need to understand in experimental error, accuracy and precision. Accuracy is how close your value or measurement is to the correct (true) value, and precision is how close repeated measurements are to each .average = sum of deviations number of measurements (1.9.3) (1.9.3) average = sum of deviations number of measurements. Then we can express the precision as a percentage by dividing the average deviation by the average value of the measurements and multiplying the result by 100. In the case of balance 2, the average value is.accuracy vs precision in chemistry The darts are grouped together and have hit the bulls-eye. This demonstrates high precision and high accuracy. Scientists always strive to maximize both in their measurements. Figure \(\PageIndex{3}\): Students in a chemistry lab are making careful measurements with a series of volumetric flasks. Accuracy and precision are . Accuracy and precision are the two important terminologies used in any measurement. These two terms describe how closely a measurement resembles a standard or known value. Accuracy defines the degree to which a measurement is close to its true value. However, precision defines how consistently the same results are obtained from .
Explains the difference between accuracy and precision by using two children's games: "Where's Waldo" and "Pin the Mitten on the Kitten". Accuracy and precision are two important factors to consider when taking data measurements. Both accuracy and precision reflect how close a measurement is to an actual value, but accuracy reflects how close a measurement is to a known or accepted value, while precision reflects how reproducible measurements are, even if they are far . The distribution of darts on a dartboard shows the difference between accuracy and precision. Assume that three darts are thrown at the dartboard, with the bulls-eye representing the true, or accepted, value of what is being measured. . Students in a chemistry lab are making careful measurements. Accuracy and precision are . The distribution of darts on a dartboard shows the difference between accuracy and precision. Assume that three darts are thrown at the dartboard, with the bulls-eye representing the true, or accepted, value of what is being measured. . Students in a chemistry lab are making careful measurements. Accuracy and precision are .Figure 1.2.1 1.2. 1: To measure the volume of liquid in this graduated cylinder, you must mentally subdivide the distance between the 21 and 22 mL marks into tenths of a milliliter, and then make a reading (estimate) at the bottom of the meniscus. Refer to the illustration in Figure 1.2.1 1.2. 1.Accuracy refers to the degree of conformity and correctness of something when compared to a true or absolute value, while precision refers to a state of strict exactness — how consistently something is strictly exact. .

This is an example of "accuracy without precision."-----ex: A student performed an analysis of a sample for its calcium content and got the following results: 14.92% 14.91% 14.88% 14.91%. The actual amount of calcium in the sample is 16.25%. What conclusion can you draw about the accuracy and precision of these results? _____ Importance of accuracy and precision in chemistry. As an analytical chemistry weight balance expert, I understand the importance of accuracy and precision in chemistry. Accuracy and precision are critical components of scientific measurement, and they play a crucial role in ensuring the quality and reliability of chemical analysis. . A chemistry tutorial designed to illustrate the difference in precision and accuracy along with a practice problem.http://www.thechemsolution.com/
Precision is a measure of how close a series of measurements are to one another. Precise measurements are highly reproducible, even if the measurements are not near the correct value. Darts thrown at a dartboard are helpful in illustrating accuracy and precision. Figure 3.12.2 3.12. 2: The distribution of darts on a dartboard shows the .Help your students consolidate their understanding of the difference between accuracy and precision using this lesson plan with activities for 14–16 year olds. In this activity, students think about an investigation to measure the boiling point of water. They decide what equipment to use, giving their reasons.
Example 1.5.3: Multiplication and Division with Significant Figures. Rule: When we multiply or divide numbers, we should round the result to the same number of digits as the number with the least number of significant figures (the least precise value in terms of multiplication and division). Divide 421.23 g by 486 mL.
In UTP cable consist of 4pair or 8 wire of different color that is used to terminate on RJ45 or 8P8C connector. Ethernet cable color coding as standardized by EIA(Electronic Industries association) and .
accuracy vs precision in chemistry|Know Your Techniques: Accuracy, Precision, and